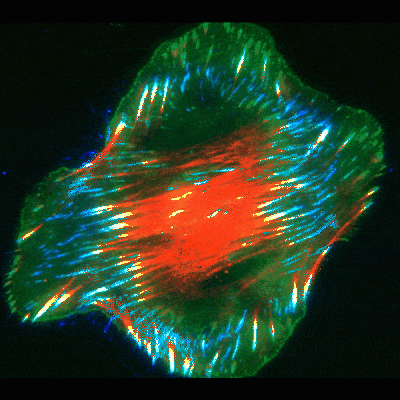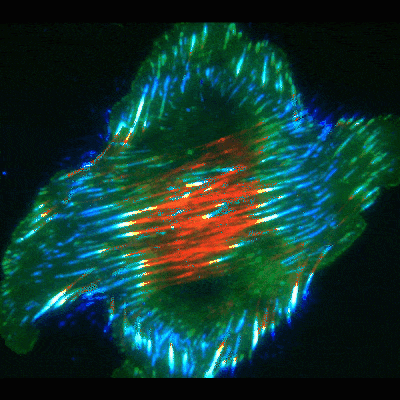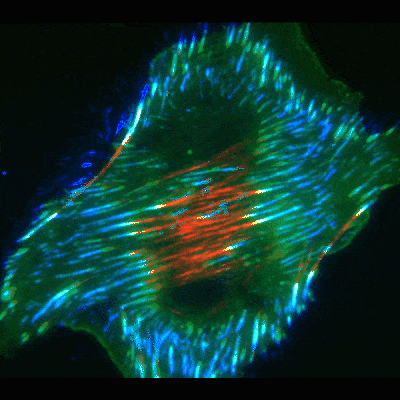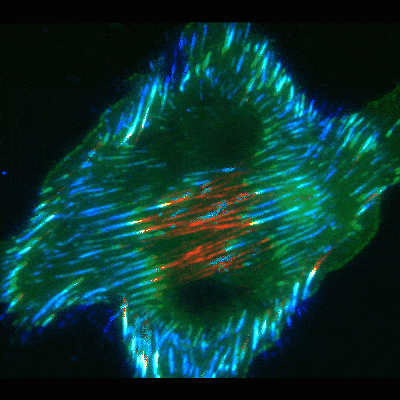
Findings provide new clues about organs’ protective barriers
In Brief:
- NIDCR scientists uncovered a new type of cellular machinery that deposits a sticky substance called fibronectin along the protective barriers that enclose organs.
- The discovery may shed light on how organs such as salivary glands form during development and how they’re sometimes breached by cancer in adulthood.
Our bodies’ tissues and organs are enclosed in linings called basement membranes, which keep their contents in and harmful ones out, including invasive cancer cells. These sheet-like membranes are a type of extracellular matrix, the 3-D, fibrous network that extends throughout the body and lends support to all our cells, tissues, and organs.
In developing and adult animals and humans, scientists have long observed that the surfaces of basement membranes are strewn with bundles of thread-like protein called fibronectin. Fibronectin has a sticky property that helps cells attach to and migrate along the underlying matrix. But no one understood how these fibrous deposits form, or why.
A recent study conducted in the lab of NIDCR’s Kenneth Yamada, MD, PhD, partially uncovers how: cellular machinery deploys contraction-like forces to pave basement membranes with fibronectin. The research by Yamada’s lab may also point to why, shedding light on how organs form during development and how protective membranes can be breached by cancer. The results were published online January 30 in Developmental Cell.
A Happy Accident
The work began by chance, when graduate student Jiaoyang Lu, PhD, looked at oral cancer cells under a microscope. He noticed dramatic accumulations of fibronectin fibers when the cells were placed on basement membranes or their extracted components. In contrast, the fibrous proteins were significantly smaller and fewer in number when the cancer or other cells were exposed to extracts of interstitial matrix—the other type of extracellular matrix more commonly known as connective tissue.
The researchers wondered how the cells were assembling the more robust fibronectin deposits. Were they using the so-called classical mechanism of fibronectin assembly or some other method? In the well-known classical mode, a cell forms a series of attachments, or focal adhesions, to the underlying matrix. These adhesions hold the cell in place while it builds a thread of fibronectin fiber by piecing individual fibronectin building blocks together, one-by-one.
To uncover the mechanism, Yamada’s team used high-resolution microscopy and fluorescent labeling to document live cells in action. As in the classical mode, these cells formed focal adhesions. But to the researchers’ surprise, many of the focal adhesions, instead of staying in place at the cell edges, slid inward towards the cell’s center, leaving behind trails of fibronectin fibers.
Focal adhesions are used by a variety of cells to attach themselves to surfaces. But these sliding adhesions seemed to be doing more than just mobilizing the cells.
“Instead of adhering the cells to the underlying matrix, these focal adhesions appear to be directly involved in fibronectin assembly,” says Yamada. “It’s a very unexpected way of using these normal attachment structures to assemble fibers of this protein.”
Looking Under the Hood
To figure out the mechanics of the process, the scientists took a closer look at the cells’ interior machinery. They focused on parts of the cell skeleton called actin cables. These wire-like components extend in straight lines throughout the cell, giving it structure and serving as conveyer belts along which objects travel to different areas in the cell.
The live-cell imaging showed that the focal adhesions were effectively reeled inward by a contraction or shortening of the actin cables, similar to how a car is towed from a ditch. In many cases, a single actin cable pulled in adhesions at each of its ends as it shortened at its center.
The scientists propose that as adhesions are pulled in from the cell edge, they tug on fibronectin just outside the cell, stretching it to expose sites where a second fibronectin protein can attach. As the inward movement continues, more pieces tack on to the growing fibronectin thread.
“We’ve identified a new type of cellular machine used by cells to pave the surface of basement membranes with fibronectin,” says Yamada. “This helps explain the long-standing observations of fibronectin deposits along the basement membranes of embryo and adult tissues—we think this is how they get there.”
Yamada speculates that fibronectin deposits may play a role in organ formation during embryonic development. Primitive cells are known to migrate along basement membranes to find where they’re meant to go. Researchers think that sticky fibronectin gives cells something to latch onto as they find their way. In adults, fibronectin might also contribute to cancer cells’ ability to breach the basement membrane to invade tissues. For example, Yamada’s lab has confirmed that fibronectin promotes oral cancer cell invasion across basement membrane-like barriers.
Yamada’s group will pursue these and other questions in future experiments. The findings could help scientists better understand how cancer becomes invasive and ultimately may lead to new treatment targets.
Related Link
Reference
Basement membrane regulates fibronectin organization using sliding focal adhesions driven by a contractile winch. Lu J, Doyle AD, Shinsato Y, Wang S, Bodendorder MA, Zheng M, Yamada KM. Dev Cell. 2020 Jan 27. pii: S1534-5807(20)30008-3. doi: 10.1016/j.devcel.2020.01.007. [Epub ahead of print]. PMID: 32004443.
Attention Editors
Reprint this article in your own publication or post to your website. NIDCR News articles are not copyrighted. Please acknowledge NIH's National Institute of Dental and Craniofacial Research as the source.
Subscribe to NIDCR Science News
Receive monthly email updates about NIDCR-supported research advance by subscribing to NIDCR Science News.