Cracking Down on a Rare Bone Disorder
A scientist’s search for a treatment
Families visit Alison Boyce, MD, at the NIH Clinical Center seeking help for their children, who often have bones that break easily and grow irregularly. Fragile bones, along with jagged dark patches on skin and signs of early puberty, are all indications of Fibrous Dysplasia/McCune Albright Syndrome (FD/MAS), a rare disorder of the skeleton, skin, and endocrine system.
"The very first thing parents do is ask what they did wrong,” says Boyce, an NIDCR pediatric endocrinologist who helps diagnose these patients. “I tell them that this is not an inherited disease, and they didn’t do anything to cause it—the disease just happens.” To these families, Boyce will become more than a long-term ally as a physician. She is also part of a scientific team that offers hope to patients by investigating better ways to diagnose and treat FD/MAS.
For as long as Boyce can remember, she loved science and wanted to be a physician. She fondly recalls drawing inspiration from the girls who assisted with science experiments on her favorite childhood TV show, Mr. Wizard’s World. Fueled by her passion, Boyce applied to Eastern Virginia Medical School in Norfolk, Va., where she received her medical degree in 2006.
After completing a residency and fellowship, Boyce joined NIDCR in 2014 as a staff clinician to study FD/MAS alongside her mentor, endocrinologist Michael Collins, MD. The research to better understand the condition began in the 1980s at NIH, and for the last 20 years, the program’s institutional home has been within NIDCR’s skeletal biology group.
FD/MAS is caused by gene mutations that arise early in development. As the embryo grows, cells carrying the mutations spread unevenly into areas that eventually give rise to the skeleton, endocrine system, and skin. Due to this patchy pattern of growth, the affected parts of the body and the severity of disease differ for every patient. When FD/MAS manifests in the endocrine system or bones, it can be disabling and painful, and can impair quality of life.
In 2016 Boyce took the lead on one of the clinical studies in the NIDCR program, working to understand the nature and course of FD/MAS and to find better ways to care for and treat patients. Since its start in 1998, the study has enrolled 300 patients ranging from ages 1 to 102. Boyce recently earned tenure-track status as an NIH Lasker Clinical Research Scholar and is now running her own lab. The Lasker program supports a small number of exceptional, early-career physician-scientists to promote their development to fully independent investigators.
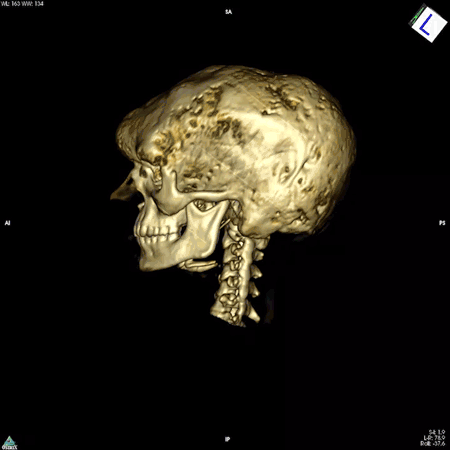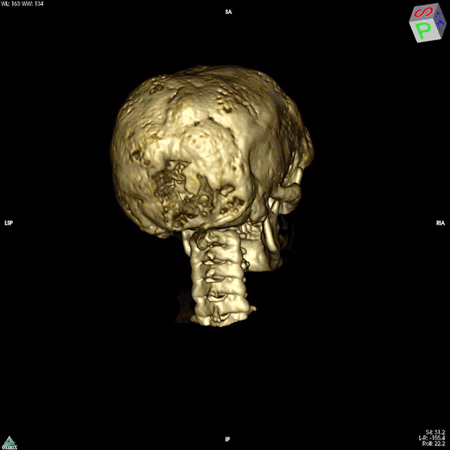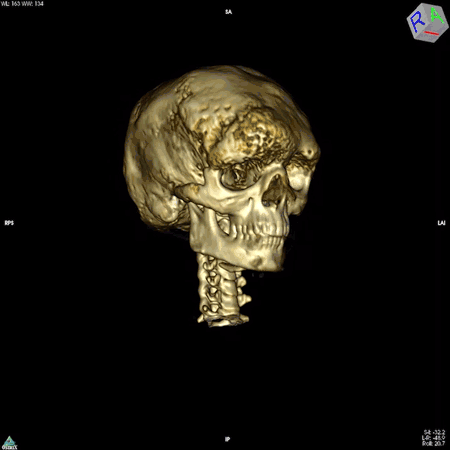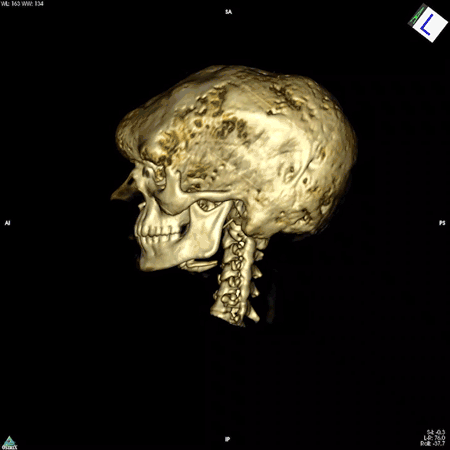
Boyce’s work focuses on patients whose bones are affected by FD/MAS. They are often born with normal skeletons, but over the course of childhood they develop scar-like fibrous tissue that replaces healthy bone. These fibrous lesions cause bones to become soft and brittle and prone to bowing and breaking, which can impair movement and cause pain. The lesions can also cause the skull bones to expand, leading to asymmetry of the head and face, and in some cases, nerve compression that causes hearing or vision loss.
Other NIH groups have developed interventions for the endocrine problems some patients experience, but “we have no treatments for the patients whose bones are affected,” Boyce says. “At NIDCR, we’ve gone a really long way trying to fill that gap.”
As part of those efforts, Boyce splits her time between the exam room and the lab. At the NIH Clinical Center, she inspects her patients from head to toe, characterizing signs and symptoms, performing bone scans, and collecting blood and tissue samples. In the lab, data from patients and animal models have helped Boyce’s team identify promising therapeutic targets, such as a protein called RANKL (receptor activator of nuclear factor kappa-Β ligand), which is abnormally elevated in FD/MAS patients.
In an ongoing clinical trial, Boyce’s group is investigating whether a drug that blocks RANKL is safe and effective in adults. Early results have been encouraging. Boyce is now hoping to launch a second trial to test whether the drug can prevent bone lesion development in children with FD/MAS.
Because the disorder has such a broad spectrum of effects, it’s hard to predict which young patients will develop severe disease. But Boyce’s research demonstrated that RANKL may serve as a useful marker to help identify high-risk patients and intervene early to prevent disease progression.
To many scientists, the complex mechanism of the disease is a fascinating puzzle to solve. However, Boyce points to the NIDCR team’s longstanding engagement with patient communities to understand their hopes and needs. “That’s why we have such a large, longstanding study at the NIH,” she says. “It’s important that the problems we’re trying to solve matter to the patients, because that’s how you really impact health.”
Related Links
References
Mosaic Effects of Growth Hormone on Fibrous Dysplasia of Bone. Roszko KL, Collins MT, Boyce AM. N Engl J Med. 2018 Nov 15;379(20):1964-1965. doi: 10.1056/NEJMc1808583. PMID: 30428295.
Activation of RANK/RANKL/OPG Pathway Is Involved in the Pathophysiology of Fibrous Dysplasia and Associated With Disease Burden. de Castro LF, Burke AB, Wang HD, Tsai J, Florenzano P, Pan KS, Bhattacharyya N, Boyce AM, Gafni RI, Molinolo AA, Robey PG, Collins MT. J Bone Miner Res. 2019 Feb;34(2):290-294. doi: 10.1002/jbmr.3602. Epub 2018 Nov 29. PMID: 30496606; PMCID: PMC6983320.
Attention Editors
Reprint this article in your own publication or post to your website. NIDCR News articles are not copyrighted. Please acknowledge NIH's National Institute of Dental and Craniofacial Research as the source.
Subscribe for NIDCR Updates
Receive email updates about the latest advances in dental, oral, and craniofacial research.
November 2024