Dialing Down Pain from the Brain
Scientist’s research could reveal new treatment targets
Four Chinese characters in calligraphy—寧靜致遠 (ning jing zhi yuan)—hang framed on Yuanyuan “Kevin” Liu’s office wall. The words from ancient Chinese literature have become the motto that Liu lives by—translated into English, they say that great achievements are not accomplished by chasing fame and fortune but by dedicating oneself to one’s calling.
Liu discovered his calling to neuroscience as a college intern at the Institute of Neuroscience in China. His interest was sparked by his mentor at the time. “He’d tell you, ‘Neuroscience is a subject where a brain studies a brain; that’s why it’s so special,’” says Liu. “I was so fascinated by the science that I decided to devote myself to the field, and now this is my career.”
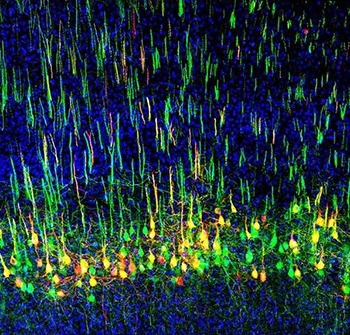
Liu, who recently joined NIDCR as an Earl Stadtman Tenure-Track Investigator, studies a bundle of nerves called the corticospinal tract, which originates in the brain’s outer layers (cortex) and projects into the spinal cord. The corticospinal tract is well-known for carrying signals that govern voluntary movements like walking and fine motor skills like grasping in apes and humans. But Liu’s research reveals that the tract controls more than just movement—it also appears to regulate pain signals from the body. These findings, Liu says, “could open up possibilities for how we might manipulate our mind to control pain. Imagine a volume control for pain.”
Liu’s first inkling of a broader role for the corticospinal tract came during his postdoc studies at Boston Children’s Hospital at Harvard Medical School. In apes and humans, nerves in the corticospinal tract project into an area at the front of the spinal cord known as the ventral horn, which relays signals to skeletal muscles to initiate movement. But in recent decades, scientists had found that some corticospinal nerves also project into the dorsal horn of the spinal cord, which receives information about touch and sensation from the body. These projections are common to all mammals. Liu wondered why part of a so-called motor tract projected to a sensory region. Could this mean the tract also plays a role in sensation?
Upon further investigation, Liu discovered that this was the case. His research showed that mice with impaired corticospinal projections to the dorsal horn have reduced responses to light touch. These animals are slow to detect tape stuck to their paws and they are less sensitive to gentle strokes from a paintbrush. By tracing signals in the nerve fibers, Liu confirmed that touch signals travel through nerves in the spinal cord and activate corticospinal neurons in the brain, which then relay the messages back to the spinal cord. This back-and-forth between the brain and spinal cord amplifies incoming light touch sensations.
Liu and his colleagues also found that this bundle of nerves plays a crucial role in mechanical allodynia, a neuropathic pain condition where light touch is perceived as painful. For people with mechanical allodynia, simple tasks like changing clothes can be challenging because of the pain triggered by fabric brushing against the skin. Liu’s team showed that eliminating certain corticospinal neurons blunted mechanical allodynia in animal models.
Liu says these results open new possibilities for treating mechanical allodynia and other types of neuropathic pain, such as trigeminal neuralgia, which is caused by damaged or irritated facial nerves.
At NIDCR, Liu is continuing his work on the corticospinal tract and other neural pathways to better understand the brain’s mechanisms for perceiving sensory information. His lab at NIDCR focuses on the bidirectional communication between the mind and body—deciphering how the body sends sensory information to the brain and how the mind controls pain. For example, in life-or-death situations, the brain temporarily shuts out pain from injuries to help us survive. Liu wants to know how our mental state can amplify or suppress the perception of sensation, which may help uncover potential targets for treating pain.
“As a scientist, the thought that I might be doing something new in the world excites me every day,” says Liu. This kind of excitement can inspire new generations of scientists, as it did for Liu as a mentee in college. As a mentor now himself, Liu draws on the lessons learned from his mentors, as well as the teachings of the Chinese philosopher, Confucius, to guide young researchers in his lab.
“Confucius’ teaching is really based on diversity, where everyone is unique and different,” says Liu. “He looks for each individual’s strength and virtue to help them fully develop. I’m a big follower of that idea as a mentor.”
Related Links
- A Four-Decade Quest to Uncover a Unique Molecule’s Secret
- When a New Immune System Attacks
- Cracking Down on a Rare Bone Disorder
References
Touch and tactile neuropathic pain sensitivity are set by corticospinal projections. Liu Y, Latremoliere A, Li X, Zhang Z, Chen M, Wang X, Fang C, Zhu J, Alexandre C, Gao Z, Chen B, Ding X, Zhou JY, Zhang Y, Chen C, Wang KH, Woolf CJ, He Z. Nature. 2018 Sep;561(7724):547-550. doi: 10.1038/s41586-018-0515-2. Epub 2018 Sep 12. PMID: 30209395; PMCID: PMC6163083.
Deconstruction of Corticospinal Circuits for Goal-Directed Motor Skills. Wang X, Liu Y, Li X, Zhang Z, Yang H, Zhang Y, Williams PR, Alwahab NSA, Kapur K, Yu B, Zhang Y, Chen M, Ding H, Gerfen CR, Wang KH, He Z. Cell. 2017 Oct 5;171(2):440-455.e14. doi: 10.1016/j.cell.2017.08.014. Epub 2017 Sep 21. PMID: 28942925; PMCID: PMC5679421.
Attention Editors
Reprint this article in your own publication or post to your website. NIDCR News articles are not copyrighted. Please acknowledge NIH's National Institute of Dental and Craniofacial Research as the source.
Subscribe for NIDCR Updates
Receive email updates about the latest advances in dental, oral, and craniofacial research.
October 2024