Building Back Broken Bones
Loss of biglycan protein impairs bone repair in mice
In Brief:
- Disrupting a sugar-laden protein called biglycan impaired bone growth and the healing of fractured bones in mice, NIDCR scientists reported recently.
- The findings point to biglycan’s importance for building strong and healthy bones and could provide insight into fracture healing and diseases marked by bone loss, such as osteoporosis.
Self-healing is an underrated superpower. Whether it’s a scraped elbow or broken toe, in most cases, our body mends itself with time. For fractured bones, this superpower is carefully orchestrated by immune cells, stem cells, and a plethora of proteins.
One such protein—a sugar-laden molecule called biglycan—plays a starring role in bone repair, according to a recent study spearheaded by postdoctoral fellow Reut Shainer, Ph.D., from the lab of NIDCR senior investigator Marian Young, Ph.D. The researchers found that biglycan is essential for bone integrity, strength, and proper fracture healing in mice. The study, published in the journal Frontiers in Physiology, may help scientists understand why some fractures heal poorly. The findings could also provide insights into diseases marked by bone loss, such as osteoporosis.
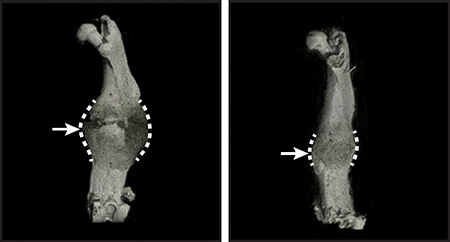
To understand the role of biglycan in bone growth and healing, the scientists bred mice that lacked biglycan. Compared to their healthy counterparts, mice born without the protein had thinner, hollower, and weaker leg bones—signs resembling osteoporosis in humans.
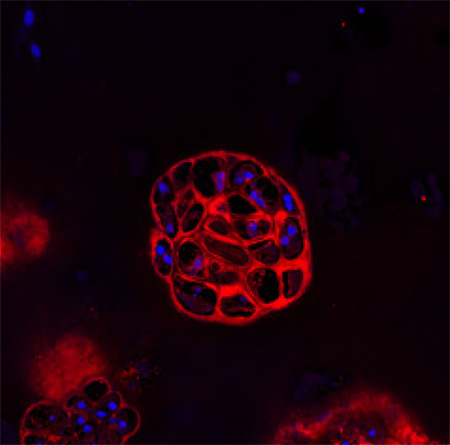
Without biglycan, the mice’s bones also healed improperly from fractures. Normally, limb bone repair starts with inflammation, which attracts skeletal stem cells from the periosteum—a thin layer of tissue around the bone—to the fracture site. The stem cells then form a mass, or callus, made of cartilage and bone that protects the fracture as it heals. Eventually, the callus is replaced entirely by new bone.
But the healing response was less robust in mice lacking biglycan. They had a weaker inflammatory response, fewer activated skeletal stem cells, and smaller protective calluses at the fracture site. Although the calluses eventually turned into bone, the structure of the new bone was irregular and less uniform, indicating that lack of biglycan can undermine bone healing.
“Bone repair is a beautiful sequence of events, and the absence of biglycan disturbs many parts of it,” said Dr. Young.
A closer look at the mice’s skeletal stem cells hinted at a possible reason. During the normal healing process, skeletal stem cells first become cartilage-producing cells, which are later replaced by bone-forming cells. But that’s not what happened in the biglycan-deficient mice. Skeletal stem cells isolated from those mice and grown in the lab developed straight into bone, skipping the formation of cartilage, which normally serves as a template for new bone growth.
These findings suggest that biglycan’s role in bone healing may relate to its influence on skeletal stem cells. Further research will be needed to understand the exact mechanisms, said Dr. Young. The findings could inform strategies to better heal fractures and treat bone disorders.
“Biglycan is very understudied in the bone field, and there is still more to learn about how it’s controlling bone growth and fracture healing,” said Dr. Young. “If you know how things work, you have a shot at fixing them one day.”
Related Links
- Therapy for Rare Bone Disorder Shows Promise in NIH Clinical Trial
- The Gut’s Role in Oral Bone Health
- Tooth Protein Prevents Bone Loss in Mice
Reference
Shainer R, Kram V, Kilts TM, Li L, Doyle AD, Shainer I, et al. Genomics and Computational Biology Core. Biglycan regulates bone development and regeneration. Front Physiol. 2023 Feb 16;14:1119368. DOI: 10.3389/fphys.2023.1119368.
Attention Editors
Reprint this article in your own publication or post to your website. NIDCR News articles are not copyrighted. Please acknowledge NIH's National Institute of Dental and Craniofacial Research as the source.
Subscribe for NIDCR Updates
Receive email updates about the latest advances in dental, oral, and craniofacial research.
June 2023