O-Glycosylation
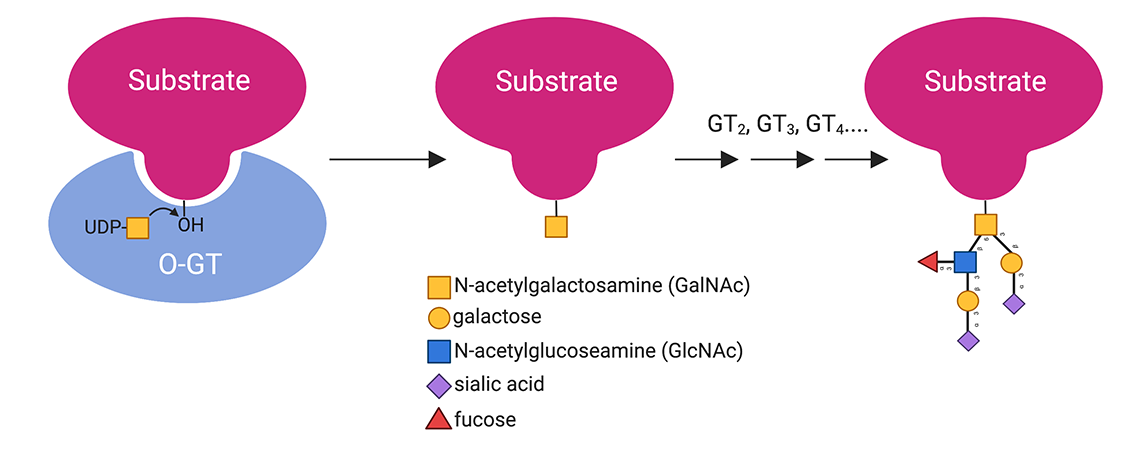
O-glycans regulate various processes throughout all domains of life. A large and diverse family of O-glycosyltransferases catalyze O-glycan biosynthesis by transferring a monosaccharide from a nucleotide sugar donor to a hydroxyl acceptor on a substrate. Our lab is interested in understanding the O-glycosyltransferases that synthesize O-glycans that modulate host:microbe and microbe:microbe interactions. We use structural methods including X-ray crystallography and cryo-EM, in combination with various biochemical, bioinformatic, and biophysical tools, to interrogate O-glycosyltransferase structure and function.
Research Projects
Mucin-type O-glycosylation: An essential modification for innate immunity and regulation of host:microbe interactions.
Polypeptide N-acetylgalactosaminyl-transferases (GalNAc-Ts) initiate mucin-type O-glycosylation by transferring GalNAc from UDP-GalNAc to Thr/Ser acceptors on protein substrates such as mucins. Densely O-glycosylated mucins, in part, give mucus that lines the epithelium gel-like properties that are important for its function in protecting underlying layers from damage and infection and modulating interactions with the microbiota.
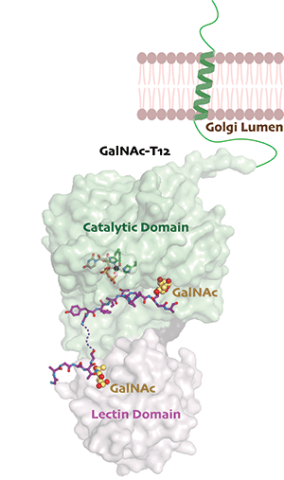
GalNAc-Ts do not recognize a consensus sequence or motif, and each isoenzyme shows unique specificity towards acceptor sites on a substrate. We are interested in understanding the diverse mechanisms used by GalNAc-Ts to recognize, bind to, and position substrates for catalysis. Our recent structural studies of the colorectal cancer (CRC) associated isoenzyme human GalNAc-T12 describe the structural basis of its unique substrate specificity and binding mode, which is mediated by a non-conserved GalNAc binding pocket in the catalytic domain of the enzyme and a conserved pocket in its lectin domain.
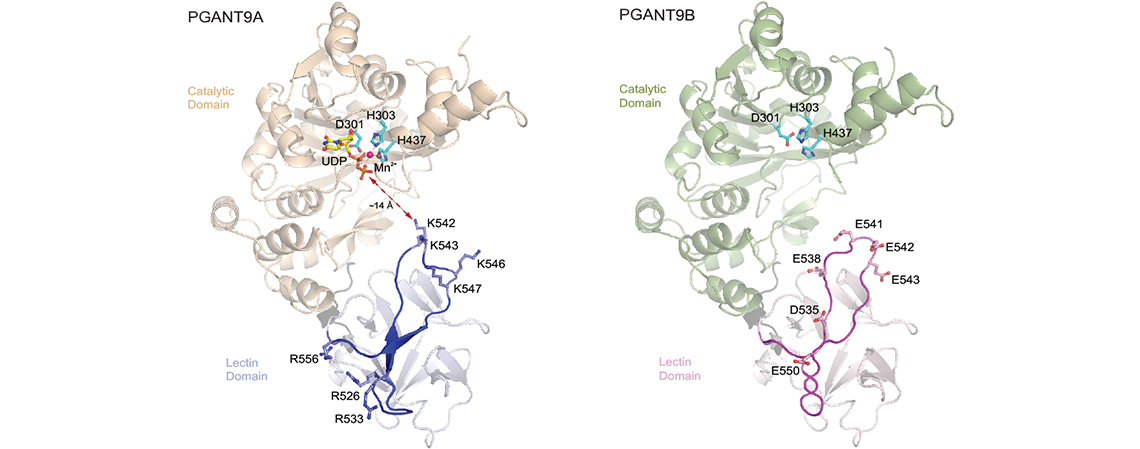
We also study how alternative splicing regulates GalNAc-T function. In collaboration with the Ten Hagen and Tabak labs at NIDCR, our studies reveal that the alternative splicing of PGANT9, a Drosophila GalNAc-T, results in 2 distinct isoforms termed PGANT9A and PGANT9B that differ in a ~30 amino acid region of the lectin domain. PGANT9A contains a net positive charge in that region, while PGANT9B contains a net negative charge. These distinct net charges dictate the divergent electrostatic-mediated substrate specificities of the 2 isoforms.
One of our goals is to use the structural and biochemical characterization of GalNAc-T substrate preferences to predict in vivo substrates, which are often unknown, and thus further our understanding of the downstream effects of aberrant O-glycosylation that arise due to mutations in GALNT genes that alter enzymatic activity. By determining GalNAc-T substrates, we can learn how aberrant glycosylation influences the protective role of mucus and its interactions with microbes in diseases such as CRC, which are associated with dysbiosis. In particular, we want to learn more about the substrate(s) of GalNAc-T12, where subsets of CRC patients have deactivating mutations in GALNT12. Overall, we hope that these studies provide insight into the specific targeting and modulation (inhibition/activation) of individual GalNAc-Ts in diseases associated with disruptions in host-microbe interactions.
Microbial glycosylation in virulence factor biosynthesis
Exopolysaccharide (EPS). Oral biofilm is comprised of rich and diverse microbial communities consisting of over 700 distinct species, and an imbalance in the composition of the community is associated with common oral diseases including caries and periodontitis and is linked to systemic disease such as colorectal cancer. Biofilm microorganisms tend to be highly resistant to antibiotics compared to planktonic ones, therefore finding new and more effective ways to control biofilm formation is critical in preventing disease. We are identifying and characterizing the O-glycosylation machinery responsible for exopolysaccharide (EPS) biosynthesis in the oral biofilm matrix. By understanding the machinery responsible for exopolysaccharide biosynthesis in oral biofilm, we can find ways to inhibit matrix formation linked to oral microbiome dysbiosis.
Capsular polysaccharides (CPS). CPS form a dense layer around particular strains of bacteria and fungi. The CPS layer can facilitate host cell entry by “hiding” other virulence factors on the bacterial surface that adhere to host cells and also can help the microbe evade the host immune response. We are particularly interested in understanding the role of CPS in pathogenic bacteria and fungi that are increasingly becoming resistant to antibiotics and are using structural and biochemical approaches to study microbial O-glycosyltransferases that synthesize CPS. Our long-term goal is to use structural and biochemical knowledge to design O-glycosyltransferase inhibitors that can function as anti-microbials.